· Expert Manufacturing
· Compliance Safeguard
· End-to-End Empowerment for Medical Innovation
· Compliance Safeguard
· End-to-End Empowerment for Medical Innovation
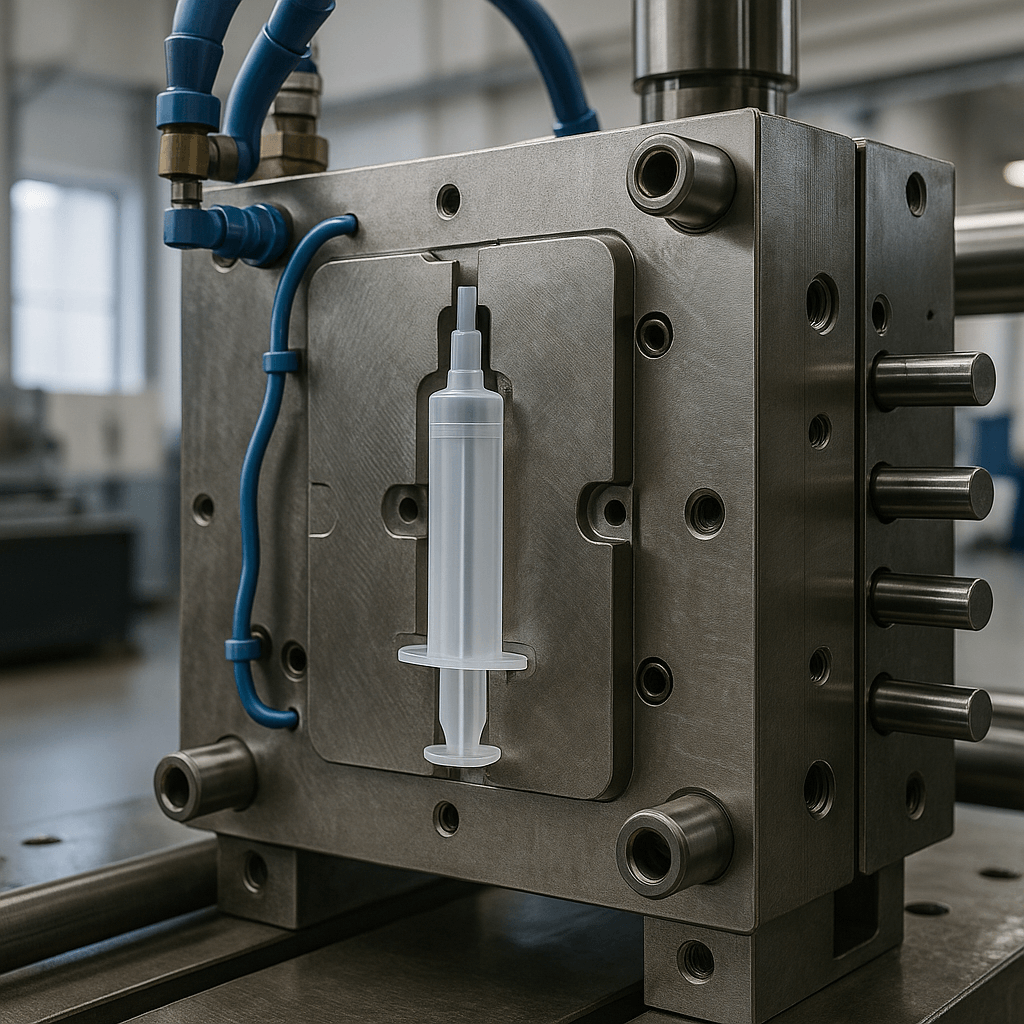
We provide global medical device companies with comprehensive OEM solutions from concept to mass production. Leveraging our ISO 13485/GMP certified systems, 10+ years of medical manufacturing expertise, and 200+ successful cases, we help reduce time-to-market by 40% and lower overall costs by 30%.
What we can do?
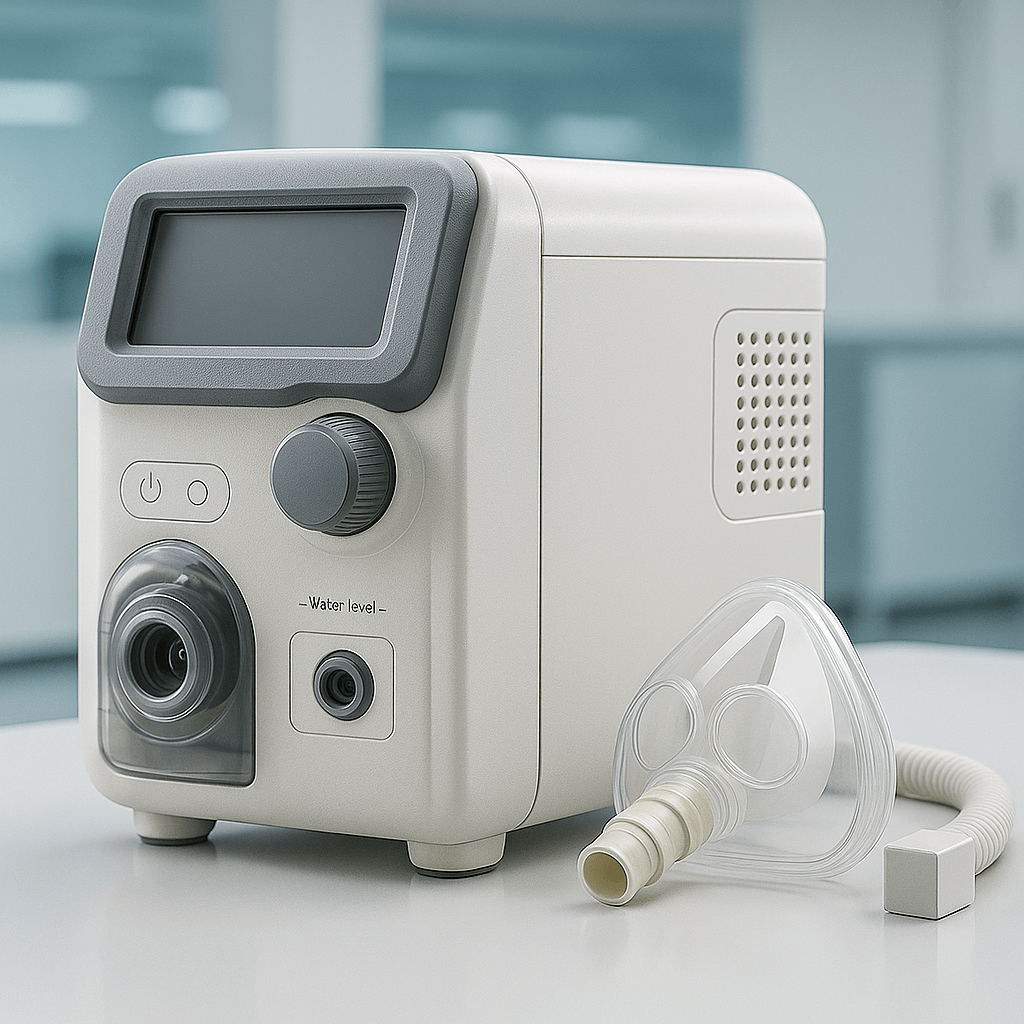
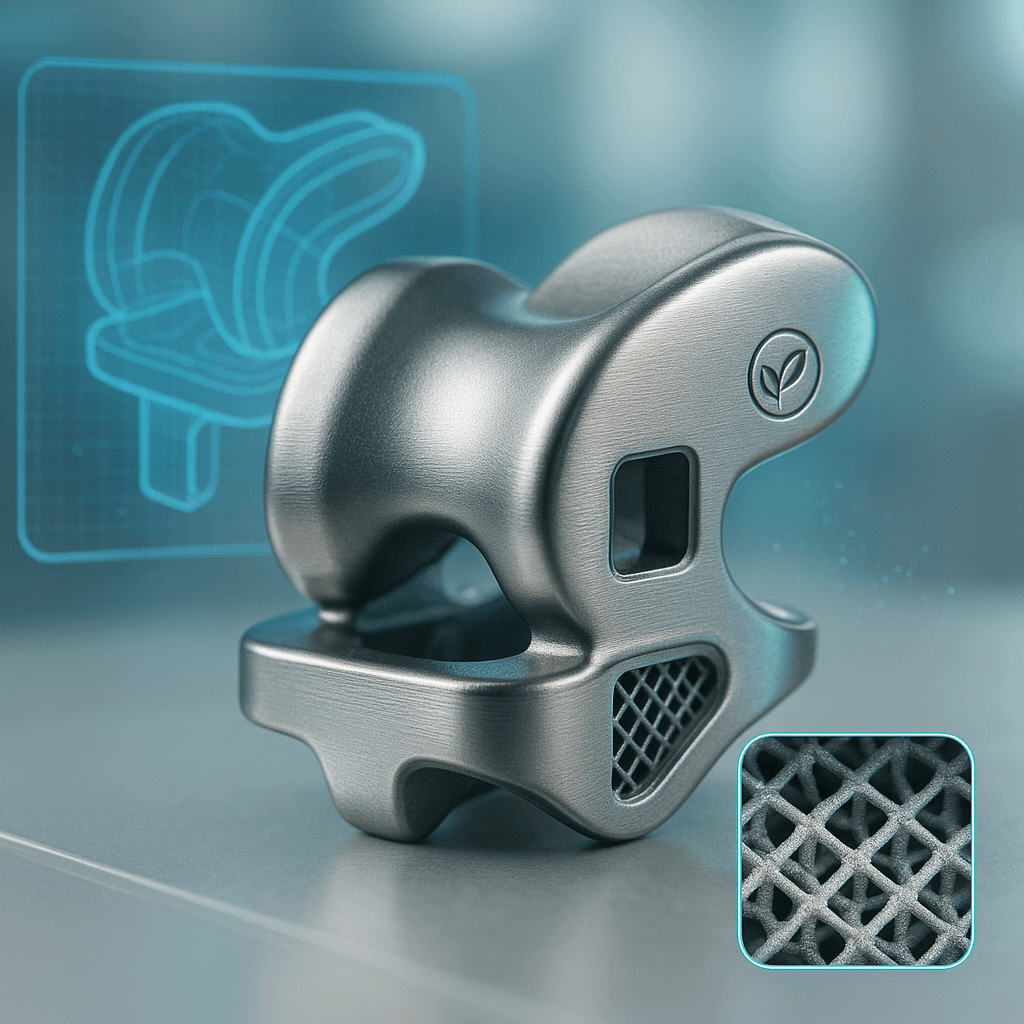
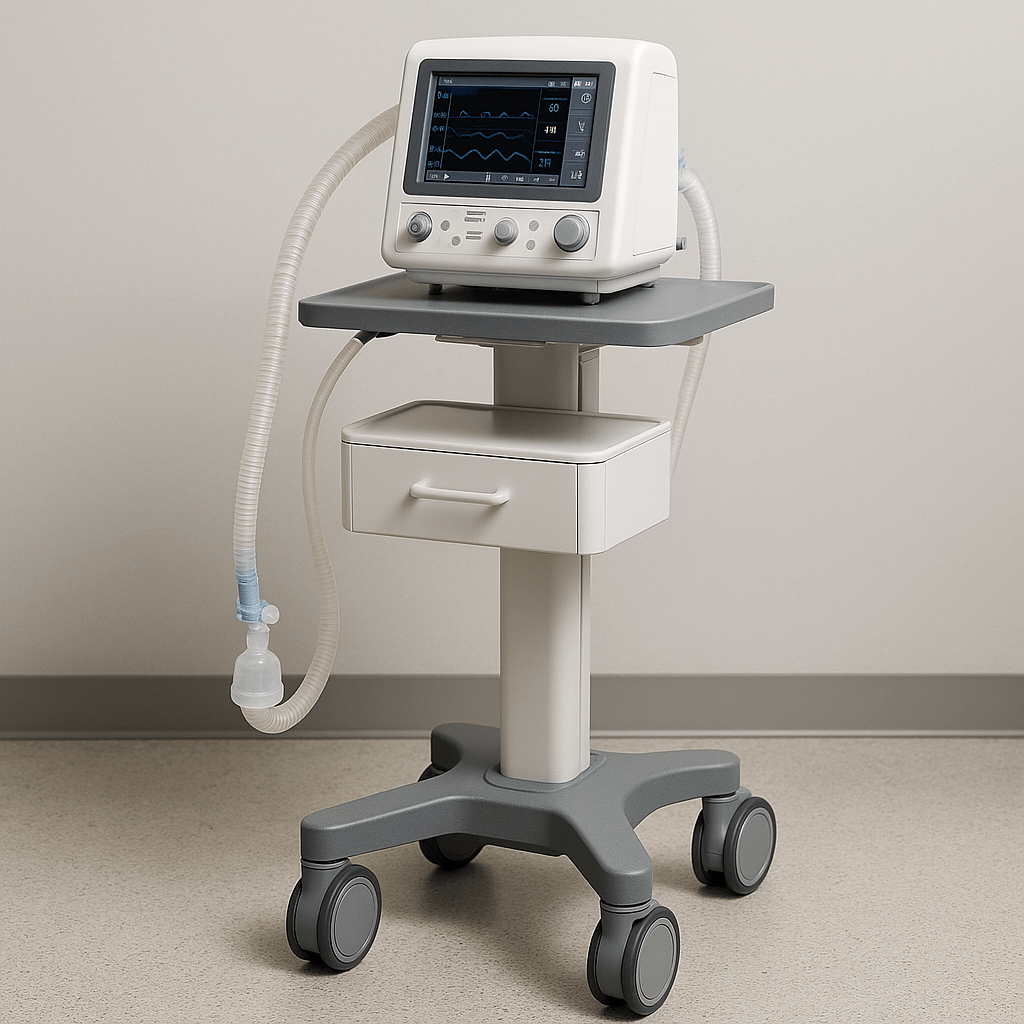
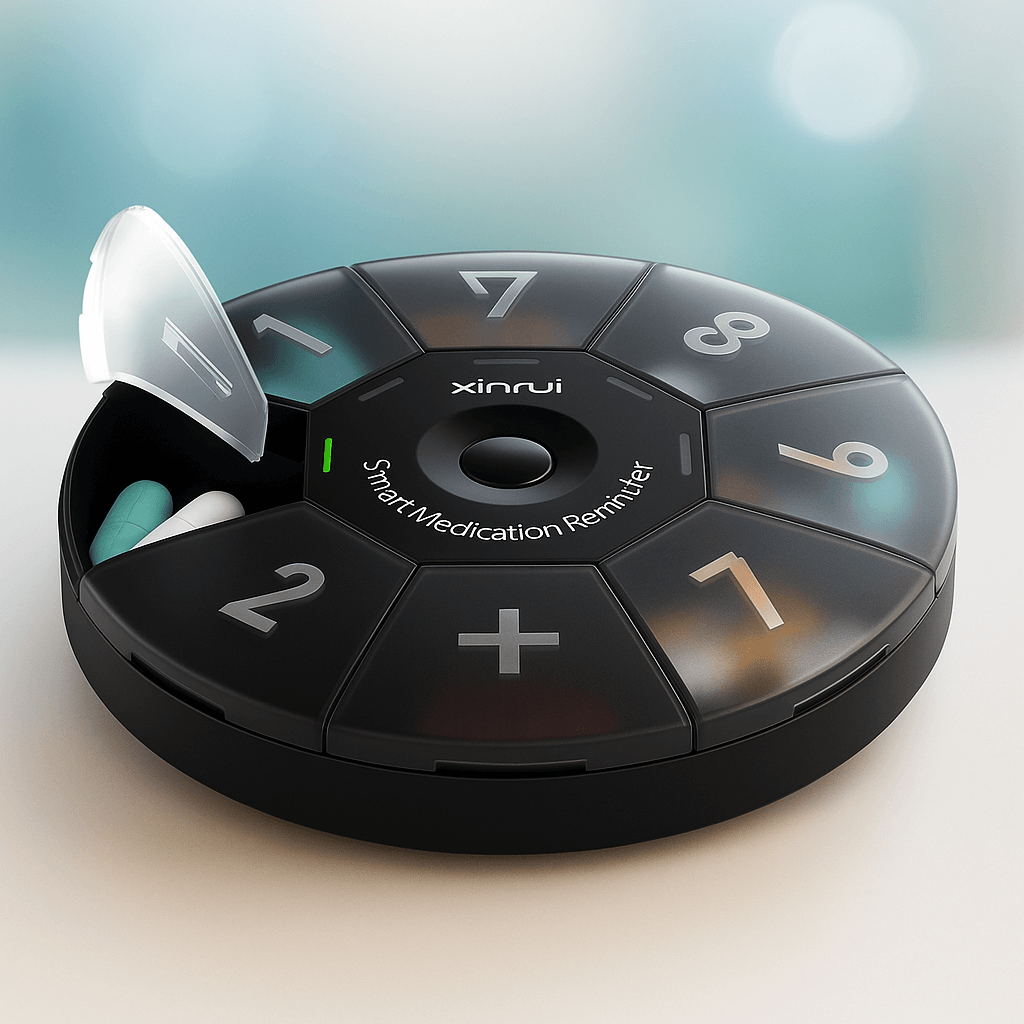
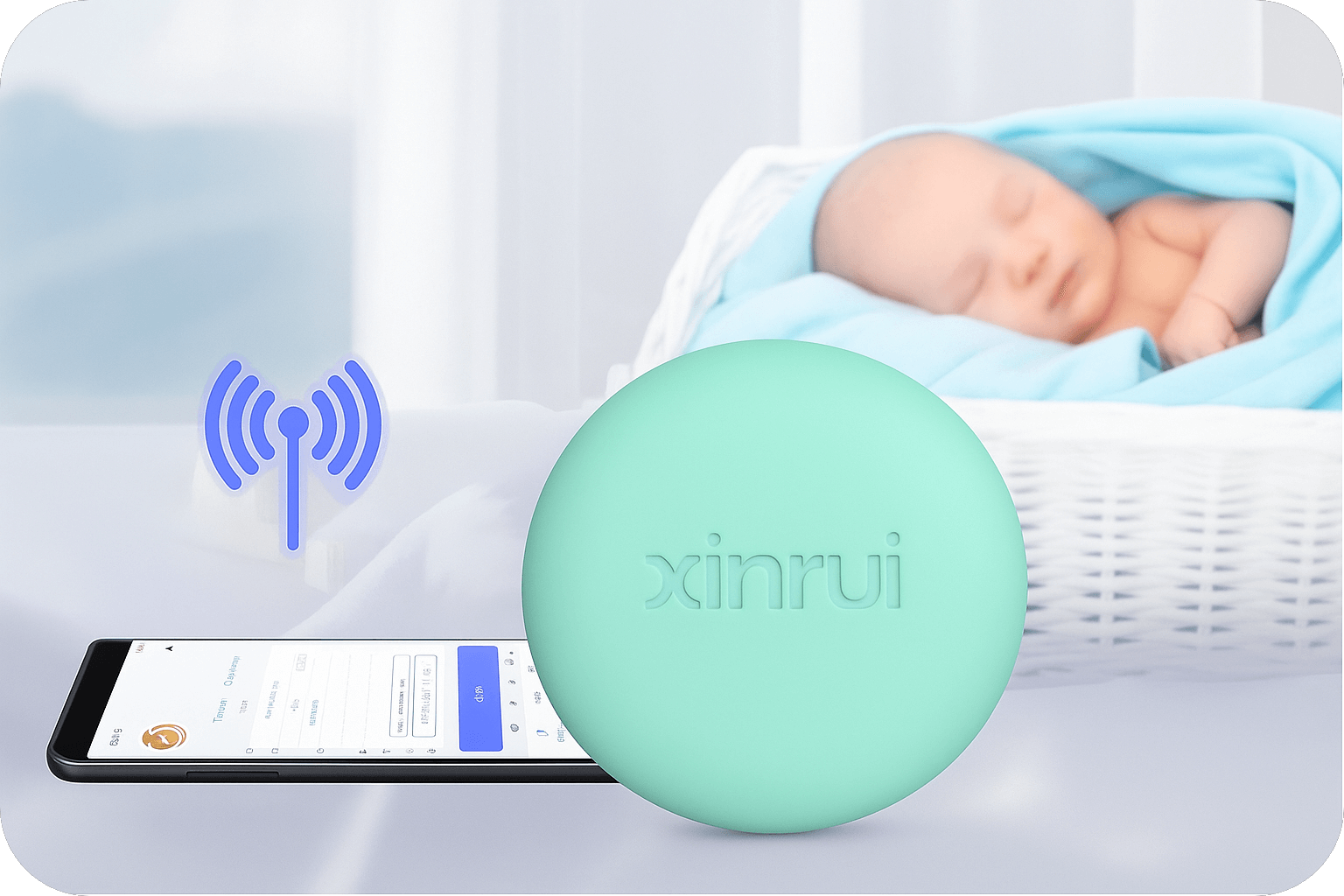
Our Own Medical Products
The common choice of global customers










FAQ

What info do you need for quoting?
Send 2D/3D files + yearly volume + surface finish needs. Quote in 3 workdays.

How long for prototypes?
We'll ship first samples in 3-7 workdays after getting your drawings (depends on complexity).

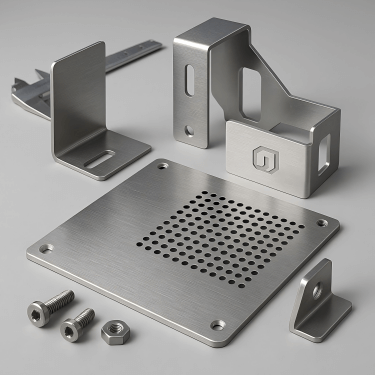
How precise is your sheet metal work?
Laser cutting ±0.1mm, bending ±0.2mm – perfect for demanding device assembly.

How do you keep defects low?
We check every critical dimension using QC plans – defect rate stays below 0.5% in production.

What's your minimum order?
Sheet metal: 50pcs. Injection molding: 500pcs (small trial runs negotiable).

What if you miss deadlines?
We cover 0.3% per day delay (in contract terms) unless it's force majeure.




 CONTACT US
CONTACT US